本文中观点不代表任何公司和组织,如有觉得有冒犯的地方,请于后台联系,我会及时删除。所有分享案例都具有孤立性,仅供参考。
受检机构/公司:Sun Pharmaceutical Industries imited
受检身份:Finished Drug Products Manufacturer-OSD
FEI号:3004561553
检查员:Pratik s Upadhyay
检查日期:12/4/2023-12/15/2023
OBSERVATION 6
Reserve samples from representative samplelots or batches of drug products selected by acceptable statistical proceduresare not examined visually at least once a year for evidence of deterioration.
Specifically, 具体来说,
A)Each lot of Controlled/Reserve(Retain) samples of drug products is not examined at least once a year for theevidence of deterioration and physical defects. Your firm's rationale based on xxxfor the selection of limited number of batches is not justifiable while thereare significant gaps identified in your firm's Quality and Production Systemsalong with several repeated product quality complaints pertaining to count variability, broken, halftablets, and lack of efficacy.计数变化、破碎、半片和缺乏功效
As per your procedure SOP018076, Titled: “Sampling,Storage, Observation and Destruction of Finished Products Control Samples”,Version: 13.0, Section: 5.4.1 “For each product, xxx batch out of totalcommercial batches packed during calendar year shall be selected for visualinspection (All pack style & counts)”. xxx batches selection per section5.4.4 for visual inspection is defined in table-1. For example, but not limitedto:
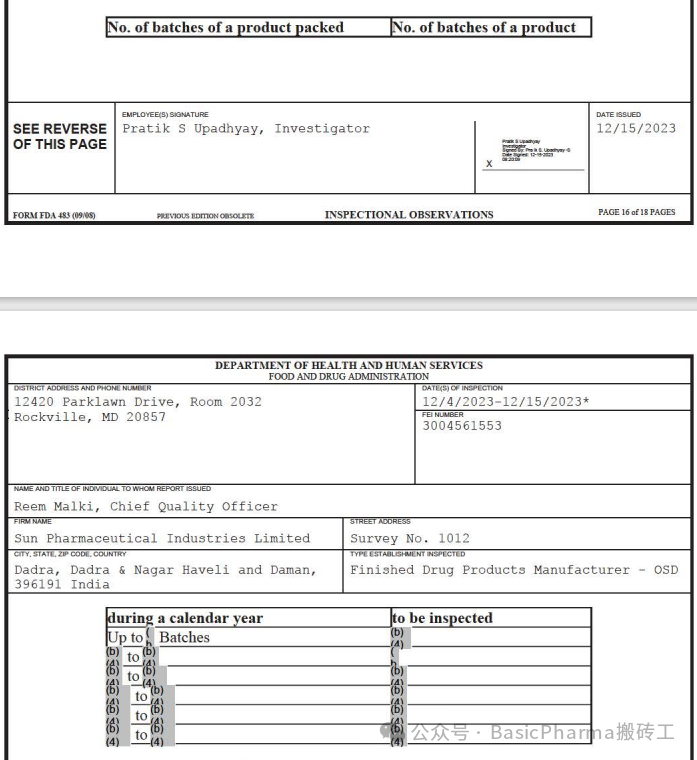
In the firm's current practices, the limited number ofbatches selected for the annual verification are the only ones that areverified throughout the products shelf life.
This reduced examination based on selection of few batcheswould be ineffective in identifying the issues pertaining to physical defectsalong with empty bottles and count variabilities until defected drug productsreach to the customers and gets reported through product quality complaints asit has been observed during many product quality complaints.
B)Your firm's Controlled/Reserve(Retain) samples examination is deficient.
Specifically, there is no provision provided in yourControlled samples logbook to record discrepancy or observations pertaining tophysical and count variability issues during annual verification. Your QA Officer(3 years in the current position) and QA Manager (10 years in current position)reported annual verification as “Ok” during each of the annual verification ofcontrolled samples since their joining. These employees stated that they havenot observed any discrepancy for any of the product's description and count(short/high) in their entire employment with the firm. Whereas your POCInvestigation Manager has observed count variabilities in controlled sampleswhile investigating complaint investigations.没有规定记录年度核查期间与物理和计数可变性问题有关的差异或观察。3年10年
西门的吐槽:说句实话,最早写一些东西,就是自己的观后感,制药的各个领域看到啥写啥,学习,回顾,总结,输出,以此加深自己的理解。所谓,日拱一卒 功不唐捐。再后来,临时被通知要迎接FDA审计,手忙脚乱下,就开始疯狂的学习各种5年来的483.我们毕竟是制药的,至少该承担自己职责范围的事情。


