本文中观点不代表任何公司和组织,如有觉得有冒犯的地方,请于后台联系,我会及时删除。所有分享案例都具有孤立性,仅供参考。
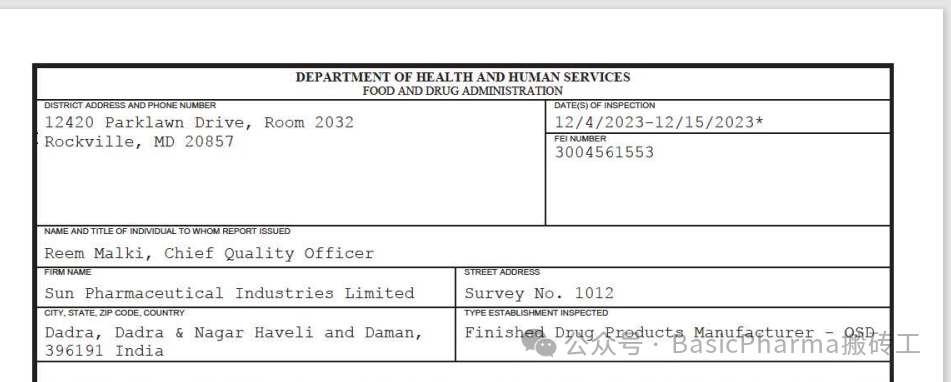
受检机构/公司:Sun Pharmaceutical Industries imited
受检身份:Finished Drug Products Manufacturer-OSD
FEI号:3004561553
检查员:Pratik s Upadhyay
检查日期:12/4/2023-12/15/2023
OBSERVATION 3
The responsibilities and proceduresapplicable to the quality control unit are not in writing and fully followed.
西门的备注:很多时候在483里quality control unit等同于质量部,另外想吐槽下,最近FDA的检查官,检查QDT qms dms tms的频率有所上升……这些系统的上线尤其需要注意。以下是部分,最近DMS发现项链接
FDA 483:系统性失效:故障分析不充分,忙所以没空调查/测试,QMS无延期流程,TMS培训不充分,系统初始评估不充分等( FDA 483:系统性失效:故障分析不充分,忙所以没空调查/测试,QMS无延期流程,TMS培训不充分,系统初始评估不充分等)
FDA 483:系统性失效:故障分析不充分,忙所以没空调查/测试,QMS无延期流程,TMS培训不充分,系统初始评估不充分等)
FDA483:上了qms和dms后混合记录仍不受控,碎纸机未受控,旧配方一年未归档,冰箱日志未登记,记录无法追溯到人( FDA483:上了qms和dms后混合记录仍不受控,碎纸机未受控,旧配方一年未归档,冰箱日志未登记,记录无法追溯到人)
FDA483:上了qms和dms后混合记录仍不受控,碎纸机未受控,旧配方一年未归档,冰箱日志未登记,记录无法追溯到人)
FDA 483 及其回复: 碎纸机、电子表格、DMS文件打印不受控,及其整改策略( FDA 483 及其回复: 碎纸机、电子表格、DMS文件打印不受控,及其整改策略)
FDA 483 及其回复: 碎纸机、电子表格、DMS文件打印不受控,及其整改策略)
FDA 483 关于文档管理系统DMS的发现项 1( FDA 483 关于文档管理系统DMS的发现项 1)
FDA 483 关于文档管理系统DMS的发现项 1)
FDA 483: 全球部署的DMS只有子公司管理员能管理子公司数据不存在的
Specifically,尤其是
A)There is a lack of Quality Unitoversight on the issuance, handling, retrieval and reconciliation of GMPdocuments that are used in the manufacturing of drug products at your site.This lack of oversight violates the firm's QA SOP 018075 for GMP form issuance.For example,GMP文件的分发、处理、检索和核对方面,质量部门缺乏监督。
西门:一般在上DMS时候,会重新梳理文件生命周期:3.上传归档(文档管理)
1)Controlled documents related to the following departments can beprinted from the Electronic Document Management System (EDMS) without anyoversight of the Quality Unit. These documents are used to record original GMPinformation. For example, but not limited to:受控文件可以从电子文件管理系统(EDMS)打印,而无需质量部门的任何监督
西门: 说句老实话,这里是表单的管理,有些公司会将表单按重要性和DI分类,最低级别是即打即用,可以由所在部门的授权人员,进行打印分发使用,这个一般也是可以认可的。但是应该存在一些管理措施。
空白表单的控制:在受监管实验室的许多程序的后面是空白表单,旨在确保符合SOP中包含的工作并收集所需数据。FDA 数据完整性指南的问题6提出了如何控制这些空白表单的问题。FDA希望此类表单的每个副本都有唯一的编号和说明。这也是对1993年出版的 FDA QC实验室检查指南中的立场的重申。“We expect raw laboratory data to be maintained in bound, (not loose or scrap sheets of paper), books oron analytical sheets for which there is accountability, such as prenumbered sheets.我们希望将原始实验室数据保存在装订好的(而不是松散或废弃的纸张)、书籍或有责任的分析表上,如未编号的表”
MHRA和世界卫生组织指导文件也提出了空白表单控制要求。此外,PIC/S和EMA监管指导文件中涵盖了这一主题。这种方法的基本原理是,不受控制的空白表单为数据造假或测试合规性提供了机会。
GMP指南中也是有相关表述,受控管理空白纸质记录和表格,例如:
制作控制:设计的记录和表格便于填写人员理解需填写的内容,并有足够的空间填写;应有唯一的版本号控制,并经过审批;主文件与副本有明显的区别,例如使用不同颜色或不同标记的纸张等,防止误用。
分发控制:受控分发空白记录和表格,确保页码连续、数量受控,例如有些公司将空白记录装订成册,每本记录印有唯一的识别号,每页记录印有页码,按识别号顺序分发和收回,并进行数量平衡的核算。
这里的观察项,是指给QA,可能发生了什么?1. 权限不受控,不是专人控制打印分发,专人专控,可以增加犯错成本,2.及时记录,下载,分发,有唯一追溯记录,形成台账,有质量人员进行追溯(文件分发平衡,线上线下很难做……一旦上量,就很考验人员质素了),这个估计没有做到
QC forms such as Form 060789: Request Form forRe-processing, Form: 039593: Dissolution analysis checklist, Form: 011918: Autosampler validation record for model EDT-08LX, Form: 034262: HPLC ChromatogramChecklist, Form 011907: U.V. Vis Spectrophotometer graph checklist.QC表格
Production forms such as Form 011406:Equipment CleaningRecord of XXX Compression Machine, Form 040690: Equipment Cleaning Record ofXXX. Form 011459:Cleaning and Operation of XXX and xxx compression machine,Form 042113: Line Clearance checklist for Product to Product Changeover.生产表格
RM warehouse forms such as Form 012373: Performance checkof Barcode scanner/RF Gu, Form 012468: Cleaning Record of Cold Storage Cabinet,Form 012472: Cleaning Record of xxx booth.RM仓库表格
Packaging forms such as Form 011450: Challenge Test forTablets Inspection machine, Form 041188: Equipment Cleaning Record TabletInspection Machine (Product to Product)包装表格
The Head of Quality Unit of your firm stated that there isno controlled copy issuance log maintained by the firm for the issuance andretrieval of GMP documents. In addition, the Quality Unit has given an accessto employees across the site to all documents on EDMS, which allows employeesof other departments to print controlled documents unrelated to theirrespective department.贵公司的质量部门负责人表示,公司没有为GMP文件的发放和检索而保持的受控副本发放日志。此外,质量部门允许整个现场的员工查阅EDMS上的所有文件,这允许其他部门的员工打印与他们各自部门无关的受控文件。
西门的吐槽:一般给到全员的下载权限上都是“仅供参考”,受控文档应当有权限隔离。权限隔离: 这个是个很容易忽视的点,与其职责无关的文件,最好能做到权限隔离,至少无法下载。权限上没法实现,就流程控制,如每个部门有专职文件控制专员负责下载分发。或者质量部有专人来负责。
2) During the inspection, I observed the Quality Unit of your firm allowed destruction of original GMP documents such as balance weight printouts, controlled forms/formats. For example, 在检查期间,我观察到贵公司的质量部门允许销毁原始GMP文件,如天平打印件、受控表格/格式。例如,
On 04-Dec-2023, I observed a balance printout pertaining toBalance ID: GPN/N/545, Dated/Timed.01-Dec-2023 xxx ,Weight: 8.007 g that wasdisposed inside your firm's main scrapyard. The Production Technician thatdisposed this printout stated the practice of disposing the balance printout isnormal by all employees in his department in the event of printing and weighingerrors.在2023年12月4日,我观察到一份有关天平ID:gpn/n/545,日期/timed.01-dec-2023XXX,重量:8.007克的天平打印输出,该打印输出被丢弃在贵公司的主废品站内。处理此打印输出的生产技术员表示,在打印和称重错误的情况下,处理天平打印输出的做法是他所在部门所有员工的正常做法。
The firm only began recording the justification and type ofdocument that was placed into the disposal bins less than one month ago on06-Nov-2023. However, reconciliation of the documents disposed of on11-Nov-2023as noted in the logbook revealed the number of pages disposed inside the binwere less than the total number of pages of the controlled forms. For example,the entry made in the logbook dated11-Nov-2023 for the disposal of CleaningChecklist - Form 037989TP-59 and Form 037989TP-60 were two separate entriespertaining to nine (9) pages forms. However, the number of pages received for disposalwere eight (8) for Form 037989TP-59 and two (2) for Form 037989TP-60. YourQuality Unit provided no justification for the missing one (1) page for Form037989TP-59 and six (6) pages for Form037989TP-60. The total of eight (8) pagesremained missing for which your firm conducted no investigation to ensure themissing pages does not get misused in the lack of Quality Unit's oversight on theissuance, retrieval, and reconciliation of GMP documents.该公司直到不到一个月前的2023年11月6日才开始记录被放入垃圾箱的文件的理由和类型。然而,如日志所述,对2023年11月11日处理的文件的核对显示,在垃圾箱中处理的页数少于受控表格的总页数。例如,日志中记录的2023年11月11日清理清单的处理条目--表格037989TP-59和表格037989TP-60是两个单独的条目,涉及九(9)页的表格。然而,表格037989TP-59收到的待处理页数为八(8)页,表格037989TP-60收到的待处理页数为两(2)页。您的质量部门没有为表格037989TP-59缺少一(1)页和表格037989TP-60缺少六(6)页提供任何理由。总共有八(8)页仍然缺失,贵公司没有进行调查,以确保缺失的页不会因质量部门对GMP文件的签发、检索和核对缺乏监督而被滥用。
B)Your QC Microbiology laboratoryhas no equipment usage logbook or any other document that records incubatorusage details for a total xxx incubators across various temperature ranges thatare used to incubate microbial and environment monitoring plates. The lack ofan issued logbook violates your SOP No.: SOP008866, Titled: “Issuance, Handlingand Retrieval of Logbook/Bounded Book ”Version No.: 8.0. Section: 5.0 and SOPNo.: SOP009405, Titled: “Instrument usage log”, Version No..3.0.Section: 5.0.
As a result of no equipment usage logbook or documentationfor the incubators, there is no assurance over its usage, breakdown (if any)and whether calibrations were performed on time. Per the above referencedprocedures, the Quality Unit of the firm is required to verify logbook entries xxxfor usage and accuracy of data entered. However, given the lack of logbooksthis verification is not happening and there was no justification provided byyour Microbiology management about not conducting these activities.由于恒温箱没有设备使用日志或文件,因此无法保证其使用情况、故障(如果有)以及是否按时进行了校准。根据上述参考程序,公司的质量部门需要验证日志条目XXX的使用情况和输入数据的准确性。然而,由于缺乏日志,这一验证没有发生,你的微生物管理部门没有提供不进行这些活动的理由。
西门的吐槽:使用日志,这没啥好说,无论是不是上了电子系统,你都应当记录日志,这是GMP中对于DI的一个保证,如果你没有记录,那有合理理由质疑数据的准确性


