链接放头前儿吧,方便你从开头看。前三弹。
https://www.bopuyun.com/pc/article/90889
https://www.bopuyun.com/pc/article/90890
https://www.bopuyun.com/pc/article/90892
除了这一节有个图,后面还有三个表,就没有图表了。
我比较喜欢一图胜千言,你呢?你觉得比利时和摩洛哥谁能赢?
4.VALIDATION AND QUALIFICATION OF WATER PURIFICATION, STORAGE, AND DISTRIBUTION SYSTEMS水净化、储存和分配系统的验证和确认
4.1Validation Requirement
Establishing the reliability of pharmaceutical water purification, storage, and distribution systems requires demonstrating control of the process through an appropriate period of monitoring and observation. Finished water is typically continuously produced and used, while product and process attributes may only be periodically assessed. The quality of bulk finished water cannot be established by only testing monograph attributes. The unit operations in the pharmaceutical water system need to demonstrate that they are in control through monitoring of the process parameters and water quality. The advent of using conductivity and total organic carbon (TOC) to define chemical purity allows the user to more quantitatively assess the water’s chemical purity and its variability as a function of routine treatment system maintenance and regeneration. Treatment processes must also demonstrate control of microbial attributes within the overall system. Some unit operations that are needed for chemical treatment may significantly increase microbial and bacterial endotoxin levels. These are later controlled by downstream unit operations. Knowledge of the treatment system processes and the effectiveness of control measures is needed to ensure that the pharmaceutical waters are acceptable for use.
建立制药用水净化、储存和分配系统的可靠性需要通过适当的监测和观察来证明对工艺的控制。成品水通常是连续生产和使用的,而产品和工艺属性可能只能定期评估。散装成品水的质量不能仅靠检测各论属性来确定。制药水系统中的单元操作需要通过对工艺参数和水质的监控来证明它们处于控制之中。使用电导率和总有机碳(TOC)来定义化学纯度的出现,允许用户更多地定量评估水的化学纯度及其作为常规处理系统维护和再生功能的变异性。处理过程还必须证明在整个系统内对微生物属性的控制。一些需要化学处理的单元操作可能会显著增加微生物和细菌内毒素水平。这些随后由下游单元操作控制。需要有处理系统流程和控制措施有效性的知识,以确保制药用水可接受使用。
Efficacy of the design, operation, sanitization, and control of the pharmaceutical water system is demonstrated through the monitoring of chemical and microbial attributes. A typical water system validation program involves an initial increased
frequency of monitoring of the treatment system process parameters and sampling and testing of major process points to demonstrate the ability to produce the acceptable water and to characterize the operation of the system. This is followed by a life cycle approach of validation maintenance and monitoring.
制药水系统的设计、操作、消毒和控制的有效性通过对化学和微生物属性的监测得到了证明。典型的水系统验证程序包括增加对处理系统工艺参数的初始监测频率,并对主要工艺点进行采样和测试,以证明生产合格水的能力,并表征系统的运行情况。接下来是验证维护和监视的生命周期方法。
4.2Validation Approach
Validation is the program of documenting, to a high level of assurance, that a specific process is capable of consistently delivering product conforming to an established set of quality attributes. A validation program qualifies and documents the design, installation, operation, and performance of the system. A graphical representation of a typical water system validation life cycle is shown in Figure 3.
验证是一种记录程序,以保证特定工艺能够持续交付符合既定质量属性集的产品。验证程序 确认并记录 系统的设计、安装、操作和性能。典型水系统验证生命周期的图形表示如图3所示。
The validation protocol should be based on the boundaries of the water system and the critical water quality and process attributes needed to maintain consistent performance. The system boundary may stop at the point of use or may include the water transfer process. If the transfer process from the distribution system outlets to the water use locations (typically either with hoses or hard-piped equipment connections) is defined as outside the water system boundary, then this transfer process still needs to be validated to not adversely affect the quality of the water as it is delivered for use. Because routine quality control (QC) microbial monitoring is performed for the same transfer process and components (e.g., hoses and heat exchangers) as that of routine water use (see 6.1.2 QC Sampling), there is some logic to include this water transfer process within the distribution system validation.
验证方案应基于水系统的边界以及保持一致性能所需的关键水质和工艺属性。系统边界可以在使用点停止,也可以包括水的转移过程。如果从分配系统出口到用水地点(通常使用软管或硬管道设备连接)的转移过程被定义为在水系统边界之外,则仍然需要验证该转移过程,以确保不会对交付使用的水的质量产生不利影响。由于常规的质量控制(QC)微生物监测与常规用水(如软管和热交换器)的转移过程和组件(见6.1.2 QC采样)是相同的,因此将水转移过程包括在分配系统验证中是有一定逻辑的。
4.2.1VALIDATION ELEMENTS
Validation is accomplished through the use of a structured, documented process. The phases of this process include Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ), and Validation Maintenance. The process is documented in a validation protocol. The elements may be in individual protocols for each phase, or integrated into variations of a DQ/IQ/OQ/PQ combined document format. The protocols are formally approved quality documents. Factory Acceptance Testing (FAT), Site Acceptance Testing (SAT), and commissioning testing of the system may supplement qualification tests for IQ or OQ provided that they are properly documented and reviewed; and if it can be shown that the system functionality is not affected by the transport and installation.
验证是通过使用结构化的、文档化的过程来完成的。该过程的各个阶段包括设计确认(DQ)、安装确认(IQ)、运行确认(OQ)、性能确认(PQ)和验证维护。该过程记录在验证方案中。这些元素可以在每个阶段的单独协议中,或者集成到DQ/IQ/OQ/PQ组合文档格式的变体中。这些方案是正式批准的质量文件。系统的工厂验收测试(FAT)、现场验收测试(SAT)和试运行测试可作为IQ或OQ的资格测试的补充,只要它们被适当地记录和审查;并能证明系统功能不受运输和安装的影响。
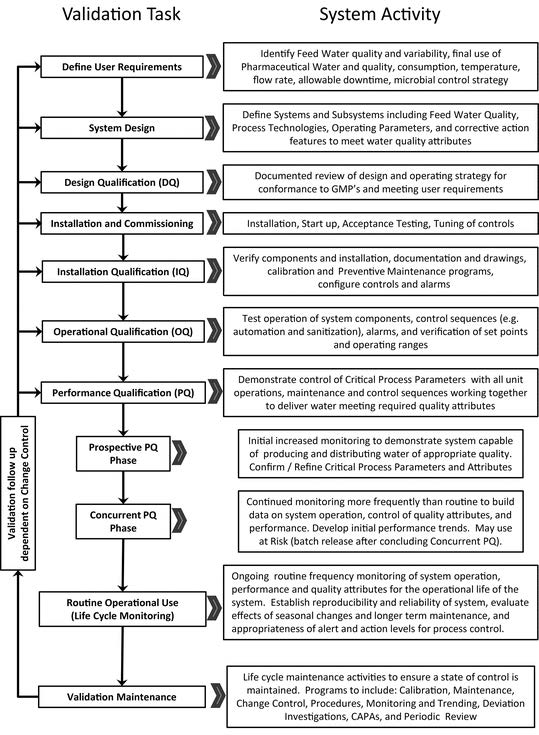
Figure 3. Water system validation life cycle. 图3.水系统验证生命周期。
4.2.2USER REQUIREMENTS SPECIFICATION AND DESIGN QUALIFICATION
The user requirements for the water system should identify the design, operation, maintenance, and quality elements needed to produce the desired water type from the available source water, including its anticipated attribute variability. The essential elements of quality need to be built in at this stage and any GMP risks mitigated to an acceptable level.
水系统的用户需求应该确定从可用水源水生产所需的水类型所需的设计、操作、维护和质量元素,包括预期属性的可变性。在这一阶段,质量的基本要素需要被纳入,任何GMP风险都要降低到可接受的水平。
The review of the specifications, system design, components, functions, and operation should be performed to demonstrate that the system complies with GMPs and verify that the design meets the user requirements. This documented review may be performed as part of the overall design process or as a separate DQ.
应对规范、系统设计、部件、功能和运行进行评审,以证明系统符合gmp要求,并验证设计满足用户要求。这种记录化的评审可以作为整体设计过程的一部分,也可以作为单独的DQ执行。
4.2.3IQ
An IQ protocol for a water system confirms that the system has been properly installed and documented. This may include verification of components, piping, installation, and weld quality; documentation of the specifications for all system components present; inspections to verify that the drawings accurately depict the final configuration of the water system and, where necessary, special tests to verify that the installation meets the design requirements. Additionally, the water system is readied for operational testing, including calibration of instruments, configuration of alarm levels and adjustment of operating parameters (e.g., flow rate, pressure).
水系统的IQ方案确保系统已正确安装并记录在案。这可能包括部件、管道、安装和焊接质量的确认;所有系统组件的规格文档;检查以确认图纸准确描述了水系统的最终配置,必要时,进行特殊测试以确认安装符合设计要求。此外,水系统已准备好进行操作测试,包括校准仪器、配置报警水位和调整操作参数(如流量、压力)。
4.2.4OQ
The OQ phase consisting of tests and inspections to verify that the equipment, system alerts, and controls are operating reliably and that appropriate Alert and Action Levels are established (this phase of qualification may overlap with aspects of IQ and PQ). During this phase of validation specific testing is performed for alarms, verifying control sequences, equipment functional checks, and verification of operating ranges. SOPs for all aspects of water system operation, maintenance, water use, water sampling, and testing, etc. should be in place and operator training completed. At the completion of the OQ, the water system has demonstrated that the components are operational and the system is producing suitable water.
OQ阶段包括测试和检查,以核实设备、系统警报和控制是否可靠运行,并确定适当的警报和动作级别(此阶段的确认可能与IQ和PQ的某些方面重叠)。在验证的这一阶段,对警报进行特定的测试,验证控制序列,设备功能检查和操作范围的核实。应制定水系统操作、维护、用水、水取样和测试等各个方面的sop,并完成操作人员培训。在完成OQ后,水系统显示各组成部分可以正常工作,并产生合适的水。
4.2.5PQ
The prospective PQ stage considers two aspects of the water system: critical process parameters and critical water attribute parameters. These are evaluated in parallel by monitoring the water quality and demonstrating acceptable quality attributes while demonstrating control of the process parameters (see 6.3 Validation Sampling Plans). The initial PQ stage may result in refinement of process parameters to yield appropriate water quality. This PQ stage includes an increased frequency of monitoring for approximately 2–4 weeks, or sufficient time to generate adequate data to demonstrate that water meeting the appropriate quality attributes is produced and distributed. One of the reasons for this duration is that biofilm, the source of planktonic organisms in water samples, takes time to develop and to determine if the sanitization unit operations and processes are adequate to control microbial proliferation. The chemical control program adequacy is typically apparent in less time than it takes to see microbial control adequacy. However, chemical purification can be compromised by poor microbial control and, to a lesser degree, vice versa.
未来PQ阶段考虑水系统的两个方面:关键工艺参数和关键水属性参数。通过监测水质和展示可接受的质量属性,同时展示对工艺参数的控制(见6.3验证抽样计划),对这些进行并行评估。初始PQ阶段可使工艺参数得到细化,以获得适当的水质。PQ阶段包括增加大约2-4周的监测频率,或有足够的时间生成足够的数据,以证明生产和分布的水符合适当的质量属性。这一持续时间的原因之一是,生物膜,浮游生物在水样中的来源,需要时间来发展和确定消毒单元操作和工艺是否足以控制微生物的增殖。化学控制程序的充分性通常比微生物控制充分性所需的时间更短。然而,化学净化会受到微生物控制不良的影响,反之亦然。
Once a level of control of microbial and chemical attributes has been demonstrated, the next phase of PQ is to continue the frequency of monitoring for approximately 2–4 weeks at a somewhat reduced level that will still give adequate data on system performance while using the pharmaceutical water. The water may be used for manufacturing at risk, and the associated products may be released only after water quality attributes have been determined to be acceptable and this validation phase has been completed. At the completion of the second phase, the data should be formally reviewed and the system approved for operational use.
一旦微生物和化学属性的控制水平得到证实,PQ的下一阶段是在稍微降低的水平上继续监测大约2-4周的频率,以便在使用制药用水时仍然能够提供有关系统性能的充分数据。这些水可以用于有风险的生产,只有在水质属性被确定为可接受且该验证阶段已完成后,相关产品才可以释放。在第二阶段完成时,应正式审查数据,并批准该系统可投入使用。
4.3Operational Use
When the water system has been placed into operational use, monitoring of the water quality attributes and the system process parameters is performed at a routine frequency (see 6.4 Routine Sampling Plans) to ensure that they remain with a state of control during long-term variability from seasonal variations in source water quality, unit operation maintenance, system sanitization processes, and earlier-established Alert and Action Levels.
当水系统投入运营使用后,以常规频率对水质属性和系统过程参数进行监测(见6.4例行采样计划),以确保在水源水质、机组运行维护、系统消毒处理过程以及较早建立的警报和行动级别的季节性变化的长期变化中,水质属性和系统过程参数保持可控状态。
The water system should continue to be monitored and evaluated on an on-going basis following a life cycle approach using online instruments or samples for laboratory-based testing. The use of online instruments and process automation technology, such as conductivity, TOC, temperature, flow rate, and pressure can facilitate improved operational control of the attributes and parameters and for process release. Manual observation of operating parameters and laboratory-based testing is also appropriate and acceptable for monitoring and trend evaluation.
水系统应继续按照生命周期的方法,使用在线仪器或实验室检测的样品进行持续监测和评价。在线仪器和过程自动化技术的使用,如电导率、TOC、温度、流量和压力,可以更容易的促进对属性和参数以及过程放行的改进操作控制。手动观察运行参数和实验室基础的测试对于监控和趋势评估也是适当和可接受的。
4.3.1MONITORING
The frequency of routine monitoring should be based on the criticality of the finished water, capabilities of the process, and ability to maintain product water quality trends. Monitoring may be adjusted from the initial validation monitoring program when there is sufficient data to support a change (see 6.4 Routine Sampling Plans).
常规监测的频率应基于成品水的关键性、工艺能力和保持产品水质趋势的能力。当有足够的数据支持变更时,可以从初始验证监视程序调整监视(见6.4例行采样计划)。
4.3.2VALIDATION MAINTENANCE
Maintaining the validated state of control requires a life cycle approach. After the completion of the PQ and release of the water system for use, ongoing activities and programs have to be in place to maintain the validated state of control after the system has been validated and placed into service (see 5.4 Operation, Maintenance, and Control). This includes unit operation, calibration, corrective maintenance, preventive maintenance, procedures, manuals and drawings, standardization of instruments, process parameter and quality attribute trending, change control, deviations, corrective and preventive actions (CAPA), training, records retention, logbooks, etc.
维护已验证的控制状态需要使用生命周期方法。在PQ完成并释放供使用的水系统后,在系统经过验证并投入使用后,必须有正在进行的活动和程序,以保持验证的控制状态(见5.4运行、维护和控制)。这包括单元操作、校准、纠正维护、预防性维护、程序、手册和图纸、仪器标准化、工艺参数和质量属性趋势、变更控制、偏差、纠正和预防措施(CAPA)、培训、记录保留、日志等。
4.3.3CHANGE CONTROL
Identification and control of changes made to unit operations and other system components, operation parameters, system sanitization, and laboratory processes or procedures need to be established. Not all changes will require validation follow up, but even minor ones, such as gasket elastomer changes could have an impact on quality attributes. The impact of the change
on process parameters and quality attributes must be identified, evaluated and remediated. This may result in a selective validation activity to demonstrate the ongoing state of control for the system and ability to maintain water quality attributes.
需要建立对单元操作和其他系统部件、操作参数、系统消毒和实验室过程或程序的变更的识别和控制。并不是所有的变更都需要后续验证,但即使是很小的变更,如垫片弹性体的变更也会对质量属性产生影响。变更对工艺参数和质量属性的影响必须识别、评估和补救。这可能导致选择性验证活动,以证明系统的持续控制状态和保持水质属性的能力。
Certain calibration and preventive maintenance activities may be considered routine tasks if they do not impact on system operation or water quality. Replacement of components needs to be carefully evaluated. Replacement of components using exact parts generally does not affect system operation or control. Replacement of components with ones that ▲are not exact parts but have▲ 1S (USP41) similar functional specifications can be performed at risk with the critical specifications (e.g., material of construction, dimensions, flow rate, response factors) having been evaluated and the differences determined to be acceptable and documented within the change control system.
某些校正和预防性维修工作,如不会影响系统运作或水质,可视为日常工作。更换部件需要仔细评估。使用确切零件更换部件一般不影响系统运行或控制。如果更换的部件不是确切零件,但功能规格相似,则可以冒着风险进行更换,前提是关键规格(例如,结构材料、尺寸、流量、响应因素)已经得到评估,并且确定差异是可接受的,并在变更控制系统中形成文件。
4.3.4PERIODIC REVIEW
The water system qualification, maintenance history, calibration records, quality and process data, issues with the unit operations and any process variability, change control, and other validation maintenance data should be assessed periodically to determine impact on the state of control.
应定期评估水系统的确认、维护历史、校准记录、质量和工艺数据、机组操作和任何工艺变化的问题、变更控制和其他验证维护数据,以确定对控制状态的影响。
The review may result in adjustments to operating or sanitization processes, calibration or maintenance plans, or monitoring plans. This may also result in additional testing or repeating certain qualification tasks (re-qualification).
评审结果可能导致操作或消毒处理过程、校准或维护计划或监控计划的调整。这也可能导致额外的测试或重复某些确认任务(重新确认)。

