本文中观点不代表任何公司和组织,如有觉得有冒犯的地方,请于后台联系,我会及时删除。所有分享案例都具有孤立性,仅供参考。
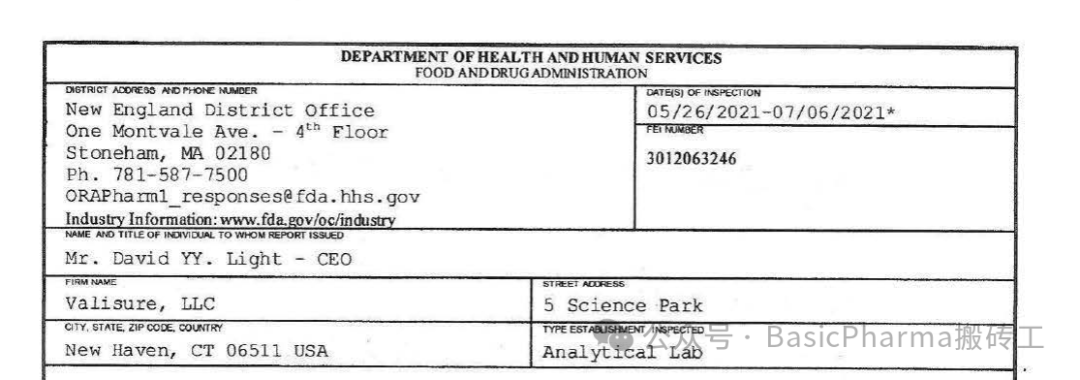
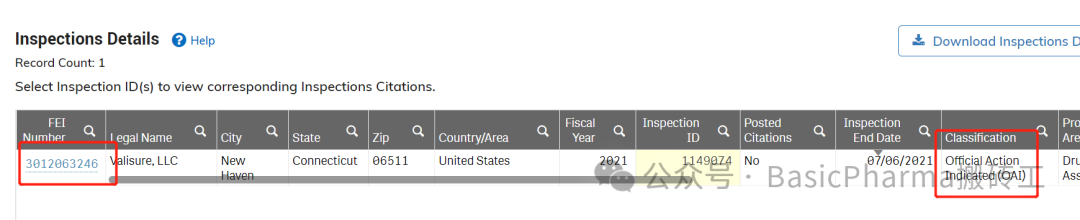
受检机构/公司:Valisure,LLC
受检地址:NewHaven,CT06511 USA
受检身份:Analytical Lab
FEI号:3012063246
检查员:Jonah S . Ufferfilge, Robert J . Martin, Sarah E. Venti
检查日期:05/26/2021-07/06/2021
披露日期:01/06/2023
检查结果:OAI
西门的吐槽
大概是前些日子,Valisure,LLC的一些检测报告,把一些事情推到的风口浪尖。
这家公司是一家美国知名的三方检测实验室,说老实话,检验方法的验证,是组成数据完整性的很重要的一环,这家公司对于分析方法与其说未经过验证,其实主要在于验证不充分以及随意更改验证状态无说明或评估,包括但不限于:
方法验证不包括准确度、精密度、特异性、定量限、范围和线性。
OOS后用未经验证的新方法检测后通过。
没有用于分析其他药品中额外杂质的文件验证流程。
不包含FDA方法或其他药典方法中概述的系统适用性要求
未经批准的分析方法(验证报告未被批准)一直被例行使用。
分析方法与药典各论不一致,无说明
以下为发现项1正文
OBSERVATION 1
The firm failed to adequately validateand/or verify analytical methods used in the evaluation of pharmaceuticalproducts in that the firm failed to document the validation/verification process,acceptance criteria, and an evaluation of the validity of the method outliningits intended use.该公司未能充分验证和/或验证用于药品评估的分析方法,因为该公司未能记录验证/确认过程、验收标准以及概述其预期用途的方法有效性评估。
For example, 比如说,
(A). Inductively Coupled Plasma MassSpectrometry (ICP-MS Test) PR-021 R1 (Verification of USP <233>ElementalImpurities):电感耦合等离子体质谱(ICP-MS测试)PR-021 R1(USP元素杂质的验证):
(a). Method Verification did not includejustification for the removal of contaminants by xxx Furthermore, there is nomention of xxx.方法验证不包括xxx去除污染物的理由。此外,没有提到xxx。
(b).Method Verification did not include anevaluation of the sample xxx fora variety of pharmaceutical dosage forms(ie.,capsules, liquids, semi-solids).方法验证不包括对多种药物剂型样品xxx的评估(即胶囊、液体、半固体)。
(c). Method Verification did not includeaccuracy, precision, specificity, limit of quantitation, range, and linearity.方法验证不包括准确度、精密度、特异性、定量限、范围和线性。
(d). Method Verification did not includethe outlined system suitability criteria. Furthermore, the ICP-MS Test does notinclude the USP<233> system suitability requirement prior to analysis. 方法验证不包括概述的系统适用性标准。此外,ICP-MS测试在分析前不包括USP<233>系统适用性要求。
(e).On 01/13/2021,the firm had analyzed asample of xxx for Elemental Impurities, the laboratory identified anOut-of-Specification result for Lead at 17.749ppb (1.7pg lead/tablet, maximumadult dose 13.ug lead/day) exceeding the firm's specification of xxx on01/14/2021, the firm had re-analyzed this sample in triplicate using a newsample preparation using xxx. The analysis confirmed the Lead results at 17.0ppb. This process of using xxx in order to rule out lead contaminated glass wasnot verified and/or validated.该公司使用xxx的新样品制备重新分析了该样品,一式三份。分析证实铅含量为17.0 ppb。使用xxx排除铅污染玻璃的过程未经验证和/或确认。
(B) Gas Chromatography Mass Spectrometry(GC-MS) impurity Test - Nitrosamines PR-018 R4 (Verification of US. FDA Method:Combined N-Nitrosodimethylamine (NDMA) and Nitrosodiethylamine (NDEA)Impurityby in Valsartan Drug Substance or Drug Product):气相色谱质谱杂质测试-亚硝胺PR-018 R4(US FDA方法:结合缬沙坦原料药或药品中的N-亚硝基二甲胺(NDMA)和亚硝基二甲胺(NDEA)杂质:
(a). Method Verification was performed onthe Valsartan Drug Product for NDMA and NDEA impurities; however, the currentmethod,PR-018 R4 is used to analyze a variety of drug products and dosage formsfor the following impurities: NDMA NDEANN-Dimethylformamide(DMF) N-Nitrosoethylisopropylaminar(NEIPA),N-Nitrosodiisopropylamine (NDIPA) and N-Nitrosomethylethylamine(NMEA)There was no documented validation process for analyzing the additional impuritieson other drug products.没有用于分析其他药品中额外杂质的文件验证流程。
(b) Method PR-018 R4 contains no systemsuitability requirement as outlined within the FDA Method. which states thecorrelation coefficient(R) of the linear calibration curves should be > 0.995不包含FDA方法中概述的系统适用性要求
(C).Liquid Chromatography High ResolutionMass Spectrometry(LC-HRMS)Impurity Test PR-020 R1, which is a Verification ofU.S.FDA Method: Liquid Chromatography -- Electrospray Ionization – High ResolutionMass Spectrometry (LC-ESI-HRMS) Method for the Determination of Nitrosamine Impuritiesin Metformin Drug Substance and Drug Product:液相色谱高分辨质谱(LC-HRMS)杂质测试PR-020 R1,这是对美国FDA方法的验证:液相色谱-电喷雾电离-高分辨质谱(LC-ESI-HRMS)法用于测定二甲双胍原料药和药品中的亚硝胺杂质:
(a) Method Verification was performed onMetformin Drug Product for impurities NDMA,NDEA,NMBA,NEIPA,N-nitrosodibutylamine(NDBA),DMF and NN-diethylformamide(DEF); however, the FDA method only listedthe following impurities NDMA, NDEA, NEIPA NDIPA, NDPA,NMPA, NDBA and NMBAThere was no documented validation process for analyzing the additionalimpurities on other drug products.没有用于分析其他药品中其他杂质的文件验证流程。
(b). There was no validation/verificationreport as of the time of the inspection (May 26,2021). The method has beenroutinely used since it's approval on 3/5/2020.没有验证/核实报告。自2020年3月5日获得批准以来,该方法一直被例行使用。
(c) Method PR-020 R1 contains no systemsuitability requirement as outlined within the FDA Method (%RSD of peak areafor each nitrosamine impurity for the first six injections NMT 10% andcumulative %RSD of the peak area for each nitrosamine impurity NMT 15%).方法PR-020R1不包含FDA方法中概述的系统适用性要求(前六次注射的各亚硝胺杂质峰面积的相对标准偏差NMT为10%,各亚硝胺杂质峰面积的累积%相对标准偏差NMT为15%)。
(D). Gas Chromatography Mass Spectrometry(GC-MS) Impurity Test- Residual Solvent PR-024 R0, which is a Verification ofUS. FDA Test Method: Direct Injection Gas Chromatography Mass Spectrometry(GC-MS)Methodfor the Detection of Listed Impurities in Hand Sanitizers: and USP <467> ResidualSolvents:气相色谱质谱(GC-MS)杂质测试-残留溶剂PR-024 R0,这是对US FDA测试方法:直接注射气相色谱-质谱(GC-MS)法;用于检测洗手液和USP<467>残留溶剂中列出的杂质;
(a) Method Verification was performed onhand sanitizers and sunscreen products for the following impurities: MethanolBenzene, Acetaldehyde and 1,1-diethoxyehtane (Acetal) however, there was noverification report(s) for the analytical activities used to analyze Benzene viaxxx under USP <467> Residual Solvents and Methanol, Acetaldehyde, and Acetalvia under the USFDA Method. The firm's method was approved for routine use onMarch 24,2021.通过分析甲醇、乙醛和乙缩醛的分析活动的验证报告。该公司的方法于2021年3月24日获得常规使用批准。
(b).Method PR-024 R0 contains no systemsuitability requirements as outlined within the FDA Method - Direct Injection,which states, %RSD of peak area for each listed impurity for all injections ofstandard solution NMT 10% and USP <467>Headspace Method ,which states SN ratiofor 1,1,1-trichloroethane in the Class 1 standard solution is NLT 5, the S/N ofeach peak in the Class 1 system suitability solution is NLT 3, and theresolution between acetonitrile and methylene chloride in in the Class 2mixture A standard solution in NLT 1.0.不包含系统适用性要求。
c).Method PR-024 R0 does not requirecalibration daily or per use. According to the firm the GC MS will be recalibratedxxx方法PR-024R0不需要每天或每次使用时进行校准。据该公司称,气相色谱-质谱联用仪将于xxx重新校准
(E).High Performance LiquidChromatography(HPLC)Test-Dosage PR-017 R3 (Verification of HPLC Dosage Test wasconducted using the USP Monograph for selected product Active Ingredient):高效液相色谱(HPLC)测试-剂量PR-017 R3(使用选定产品活性成分的USP各论对HPLC剂量测试进行验证):
(a). The HPLC Method for DextromethorphanSoft Gels Capsules was inconsistent with Dextromethorphan Bromide USPMonograph. The USP Monograph requires that the Mobile Phase be Docusate Sodiumand Ammonium Nitrate in Acetonitrile and water (70:30); the UV Detector be setat 280nm; and the system suitability with a tailing factor of NMT 2.5 and RSD NMT2.0% for the standard solution. However, the firm’s method uses a Mobile Phase xxxand there is no system suitability requirement. There was no documentedvalidation process for these changes to the USP Monograph. This method was usedto analyze xxx Dextromethorphan Soft Gel Capsules (lot number xxx)USP各论不一致280纳米该公司的方法使用流动相xxx,并且没有系统适用性要求。USP各论的这些变更没有记录验证过程。该方法用于分析xxx右美沙芬软胶囊(批号xxx)
(b) The HPLC Method for FexofenadineHydrochloride Tablets was inconsistent with USP Monograph in that the firm usedan xxx for Fexofenadine Hydrochloride Tablets and the method does not have asystem suitability requirement. The firm failed to verify equivalency- of thexxx and HPLC. Furthermore, according to the firm, analysts will conduct xxx inorder to demonstrate the system is suitable for a run. This method was used toanalyze xxx Allergy Relief Tablets (lot numbers xxx and xxx.与USP各论不一致该方法没有系统适用性要求等效性

最近又又又中招流感了,上海每次流感必中招,这波反应特别剧烈,躺了两三天…
养养花,peace and love


