本文中观点不代表任何公司和组织,如有觉得有冒犯的地方,请于后台联系,我会及时删除。所有分享案例都具有孤立性,仅供参考。
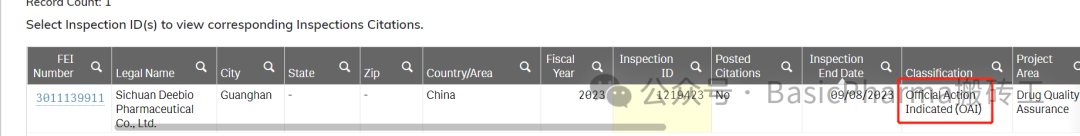
2023.09.04-2023.09.08 FDA 对四川一家药企进行了审计。
类型:API
检查结果:OAI
检查员:Asren Karapetyan,Anders W Evenson
在大年三十的这一天,FDA更新了一些检查结果,也披露了一家四川药企的483。纠结了下,还是分享下。其实对于审计而言,最重要的不是你具体做的怎么样,要注意回答,所有的必须能呈现,不然很麻烦.

OBSERVATION 1
Laboratory records are not completed contemporaneously and do not include complete data derived from all tests to assure compliance with established specifications and standards. Specifically, 实验室记录不是同时完成的,不包括从所有测试中获得的完整数据,以确保符合既定的规范和标准。具体来说,
A.During our visit to your QC microbiology laboratory, we observed that microbiology TAMC test results for QC microbiology work bench (xxx plates)and bio safety cabinet (xxx plates), xxx water samples from Workshop xxx ( xxx plates , sample points xxx USP drug substance production for US Market), and xxx samples from Workshop xxx plates, sample points xxx (domestic and non US international market production), and Workshop xxx(xxx plates , sample points xxx)( (domestic and non US international market production) purported to be read by your QC team leader, had not been recorded per your firm's SOP no. MA0057-04, titled Data reliability management procedures’, effective date 07/01/2021. Specifically, upon entering the microbiology laboratory on 09/04/2023 at approximately 11:40 am, we observed approximately xxx plates in the waste bin which had been xxx. Per your QC team leader, she stated that she had read these plates earlier in the morning around 10:00 am, and the test worksheet with recorded results was downstairs with a QA personnel. Your QC team leader than proceeded to provide misleading information, stating that she did not read the results as she had stated doing so when we first arrived in the laboratory, and shortly after this, stated that she did read the results and signed the test data worksheet which was somewhere on the xxx floor with QA personnel, only to finally admit that she did in fact read the plates, however, was not telling the truth about recording the results on respective data worksheets, and no worksheet existed. When we asked the analyst how she remembered the results of all xxx plates. she stated that it was in her “mind” and proceeded to provide at least xxx plate results varying from 18 to 23 CFU from "memory''. Your firm's investigation was not able to replicate any of these 12 values that your analyst provided to us in person. We left the laboratory around 1:40 pm, having initially arrived around 11 :40 am. As a result of providing misleading information with respect to these samples, your firm delayed our inspection. 在我们访问贵公司的QC微生物实验室期间,我们观察到QC微生物工作台(xxx板)和生物安全柜(xxx板)的微生物TAMC检测结果、来自xxx车间的xxx水样(xxx板,样品点xxx美国市场USP原料药生产)以及来自xxx车间xxx板、样品点xxx(国内和非美国国际市场生产)和xxx车间XXX(XXX板, 样品点xxx)((国内和非美国国际市场生产)据称由贵公司质量控制团队负责人读取,但未按照贵公司的SOP编号MA0057-04“数据可靠性管理程序”进行记录,生效日期为2021年7月1日。具体来说,在2023年09月04日上午11:40左右进入微生物实验室时,我们在垃圾箱中发现了大约xxx个板子,这些板子曾经是xxx个。根据你的QC小组组长的说法,她说她在上午10:00左右对这些样品进行了读板,记录了结果的测试工作表在楼下的QA人员处。然后,你们的QC小组组长继续提供误导性信息,声称她没有阅读结果,因为她在我们第一次到达实验室时已经这样做了,此后不久,她声称她确实阅读了结果并与QA人员在xxx层的某处签署了测试数据工作表,只是最终承认她确实阅读了样品板,但是,关于在各自的数据工作表中记录结果一事,她没有说实话,并且不存在工作表。当我们问分析师她如何记住所有xxx板的结果时。她表示这是她的“想法”,并根据“记忆”提供了至少xxx个从18到23 CFU不等的板结果。贵公司的调查无法复制您的分析师亲自向我们提供的这12个值中的任何一个。我们在下午1:40左右离开实验室,最初在上午11 :40左右到达。由于提供了有关这些样品的误导性信息,贵公司推迟了我们的检查。
B.During our review of xxx USP drug substance test item Limit of xxx 'performed with SevenExcellence software on your Multiparameter analyzer equipment ID No.J01-0310A. we observed no available electronic test data for process validation batches xxx which were subsequently shipped for the US Market after QA approval. Per your firm, the electronic data had been lost due to potential inadequate data backup procedures. Next, when we requested the related physical analytical batch records, we observed no printouts attached with the test worksheets reviewed by QC and QA for batch approval and release for batches xxx Per your firm, at the time of performing these tests, the printer was not connected, and no such printout exists for any test performed prior to June 2022. There is no evidence of xxx testing performed for these process validation batches, which have been shipped to the US Market 在我们使用SevenExcellence软件对您的多参数分析仪设备(ID号J01-0310A)进行的xxx USP原料药检验项目限值XXX‘的审查期间。我们没有观察到工艺验证批次xxx的可用电子测试数据,该批次在质量保证批准后随后运往美国市场。据贵公司称,由于可能的数据备份程序不充分,电子数据已经丢失。接下来,当我们要求相关的纸质检验批记录时,我们发现QC和QA审查的批次xxx批批批准和放行的测试工作表中没有附带打印输出。在进行这些测试时,打印机未连接,2022年6月之前进行的任何测试都没有此类打印输出。没有证据表明对这些已运往美国市场的工艺验证批次进行了xxx测试
C.During my review of electronic test data for test item Limit of xxx with specification as “limit ofre-tested until desirable results are achieved. The original test results for batch no.xxx (forced degradation) and batch no.xxx (accelerated 2-month stability study) were both O0S. These results were not reported, and no laboratory investigation was initiated per your firm's OOS procedure. For Example, sample for batch no. xxx was tested on 11/01/2021 at around xxx pm with result as xxx%. This result was not reported, and no OOS investigation was initiated. Shortly after on the same day around xxx pm, a second test was performed with result of xxx %, which was within specification and reported. 在我审查质量标准为“限值小于xxx”的测试项目限值xxx的电子测试数据期间,我们的审查发现,当在xxx USP原料药分析过程中遇到不良结果时,将重新测试样品,直到获得理想结果。批号xxx(强制降解)和批号xxx(加速2个月稳定性研究)的原始测试结果均为0。没有报告这些结果,也没有根据贵公司的OOS程序启动实验室调查。例如,批号为xxx的样品于2021年11月1日下午xxx点左右进行测试,结果为xxx%。没有报告这一结果,也没有启动OOS调查。不久后,在同一天下午xxx点左右,进行了第二次测试,结果为xxx %,在规定范围内并已报告。
以下仅供参考
西门:这里我唯一想说的,Sevenexcellence,应该只是HMI屏幕,不属于一个独立软件。其实还是小型设备(如ph计、天平、粒子计数器等)的一个数据管理。这里其实并不确认实际是什么情况。但是,一定要做的是,商业化生产,最好配个打印机,并在内部文件中定义好official record是什么。
PIC/S PI-041 section 8.9: 8.9 Direct print-outs from electronic systems 8.9.1 Some very simple electronic systems, e.g. balances, pH meters or simple processing equipment which do not store data, generate directly-printed paper records. These types of systems and records provide limited opportunity to influence the presentation of data by (re-)processing, changing of electronic date/time stamps. In these circumstances, the original record should be signed and dated by the person generating the record and information to ensure traceability, such as sample ID, batch number, etc. should be recorded on the record. These original records should be attached to batch processing or testing records. 8.9.2 Consideration should be given to ensuring these records are enduring (see section 8.6.1).
综上,PICS其实是个显著的主流代表,还是上系统为主。但是PICS比较好的地方,忽略了ph计的数据暂存这一点。答案定义此类储存是暂时的,进行一个数据完整性评估,并有相应输出。如果它不永久存储数据,您就没有需要管理的电子记录。在这种情况下,纸质报告(或将显示值手动转换为纸质记录)是正式记录。许多pH计、渗透压计和分析天平都属于这一领域。关于实施四眼原则(“第二人复核”)的变化是指使用计算机化系统而不是第二双眼睛手动通过USB备份到移动截至并传输到一个受控的安全存储位置(注1:在创建、处理或存储GxP数据的计算机上,默认情况下应该禁用USB端口。传输数据的临时介质media、数据来源和目的地址(位置或链接,如果是电子日志) 传输文件的数量和大小、迁移/备份的日期和时间、数据传输一致性的确认、根据需要验证从源位置删除的数据、不可避免地,继续使用当前的但略显陈旧的技术会有一些风险-这些风险的跟踪,最好都记录下来,这样挑战会小。而且尤其注意,迎审的时候,要自信,条理清晰(最好有自己的故事线,并在war room跟辅导者 确认好策略)进行针对性回答,把自己所知,所能做的回复出来。


