本文中观点不代表任何公司和组织,如有觉得有冒犯的地方,请于后台联系,我会及时删除。所有分享案例都具有孤立性,仅供参考。
2023.02.06-2023.02.17 FDA 对印度Cipla公司一个工厂进行了审计。(2023.03.02披露483,2023.11.17发送警告信)
检查官:Saleem A Akhtar, Jose E Melendez
此前有读了其警告信和发现项1(见谅全文错别字 灌装……不影响中文阅读)
FDA警告信:CIPLA药业的无菌模拟罐装污染、环境检测方案无由更改 及其建议项( FDA警告信:CIPLA药业的无菌模拟罐装污染、环境检测方案无由更改 及其建议项)
FDA警告信:CIPLA药业的无菌模拟罐装污染、环境检测方案无由更改 及其建议项)
今天分享的是发现项2.A
OBSERVATION 2
Procedures designed to prevent microbiological contamination of drug products pmporting to be sterile are not established, written and followed.未建立、编写和遵循旨在防止无菌药品微生物污染的程序。
Specifically, manufacturing process simulation procedures designed to prevent microbiological contamination for the drug products purporting to be sterile are deficient to ensure sterile drugs manufactured in xxx are safe and effective. For example:具体而言,旨在防止声称产品微生物污染的无菌工艺模拟程序不足以确保在xxx生产的无菌药品安全有效。例如:
A. The firm manufactures sterile solutions and suspensions for the US market in xxx filling lines by using xxx machines in xxx. The xxx area xxx is maintained as a Grade A area that is xxx a Grade C area. During filling operations some portion of the xxx (about xxx mm) are exposed to the Grade C area when the xxx. Since January 2021. during batch manufacturing there were 8 instances when power failure occurred in the Grade C area that surrounds the Grade A area as follows:该公司在xxx使用xxx机器在xxx灌装线为美国市场生产无菌溶液和悬浮液。xxx区域xxx保持为A级区域,即XXX C级区域。在灌装操作期间,xxx的一部分(约xxx毫米)暴露在C级区域。自2021年1月以来。在批生产过程中,围绕A级区域的C级区域发生了8次电源故障,具体情况如下:
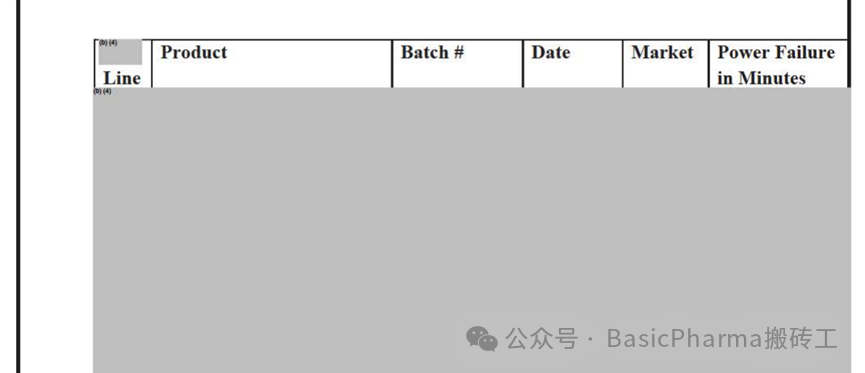
During power failure, the filling machine stops however, the HVAC system in Grade Area xxx keep running as it xxx. However, HVAC system in Grade C area does not work during power failure. As per the firm's Aseptic Process Validation Protocols for Solutions and Suspension (# FN P/APV/01, Version: 27 and FNP/APVSM/01, Version: 10 respectively) a power failure is not considered an intervention and is not carried out during aseptic process simulation (media fill) studies despite the fact that power failures during routine manufacturing require xxx cleaning, which is considered the worst case intervention. The firm does not have scientific data established through process simulation studies to fully understand the effect that power failures have on the manufactured batch.在电源故障期间,灌装机停止运行,但是xxx级区域的HVAC系统保持运行。但是,C级区域的HVAC系统在停电期间不工作。根据公司的溶液和悬浮液无菌工艺验证方案(# FN P/APV/01,版本:27和FNP/APVSM/01,版本:10),停电不被视为干预措施,并且在无菌工艺模拟(培养基灌装)研究期间不进行电源故障验证,尽管在常规生产过程中电源故障需要进行xxx清洗,这被视为最坏的干预措施。该公司没有通过过程模拟研究建立的科学数据来充分了解电源故障对生产批次的影响
西门:任何可能影响产品质量的供电中断,无论多么短暂,都属于生产偏差,必须包含在批次记录中(211.100、211.192)。2,再来谈停电干预。
2.1 一般情况下这里比较有趣的情况是,灌装设备停电,HVAC有点,也就是所谓的,环境还在。而且很神奇的事情是,这家公司的灌装机停电过于频繁,以至于变成了类似于”机械故障“.The performance of interventions should be accomplished by qualified personnel, including maintenancepersonnel, following defined procedures. The ability of the operator/mechanic to intervene in the process to fix a "mechanical failure" should be reflected in the APS. For ex- ample, a firm may choose to simulate an equipment breakdown. However, it is difficult to predict the frequency of occurrence of breakdowns, part replacements, or other non-routine corrective interventions. If these types of corrective interventions do not occur naturally during a process simulation study, the activities associated with them must be simulated to qualify their performance during routine operations.干预的执行应由有资质的人员,包括维修人员,根据明确的规程执行。工艺过程中,操作者/机修工干预维修“机械故障”的能力应在APS中反映。例如,公司可模拟一次机械故障。但很难预测故障、换件、或其他非日常的非常规干预发生的频率。若在工艺模拟研究中这些类型的非常规干预不在自然条件下发生,必须对其进行模拟以确保日常运作的合格执行。(PDA TR22)
2.3 停电干预的细节
Media fill studies include media preparation/compounding, filtration, filling, any other additionalaseptic process. High risk aseptic operations should not be justified with successful media fill. e.g.transfer of open sterile contact parts/components in grade B without Grade A continuity, leakage postto sterile filter, power failure of Grade A air supply system etc. should not be justified by simulating inmedia fill. 培养基灌装研究包括培养基制备/混合、过滤、灌装以及任何其他额外的无菌工艺。高风险无菌操作不应以成功的培养基灌装为理由。例如,无A级连续性的B级开放式无菌接触部件/组件的转移、无菌过滤器的泄漏柱、A级供气系统的电源故障等。不应通过模拟媒体填充来证明其合理性。此外,比较有意思的是,在同一份文件中也有对机械故障的注释,外部故障:由于非工艺事件导致的不符合验证验收标准的故障。例如电源故障或设备故障。Extrinsic failure : A failure to meet qualification or validation acceptance criteria resulting from nonprocess occurrences. E.g. power failures or equipment failures.
其实APS中模拟停电(这里指的B级咯)也屡见不鲜,例如2021 ISPE 无菌会议监管小组,MHRA 首席GMDP检察员Alan Moon也吐槽过这点。

艾伦:试图验证不良做法的概念是我们经常看到的,而不是我们想要的。例如,在无菌模拟灌装中加入干预并不能解决问题。我有时看到有人模拟停电。
2.4 个人观点
所以停电干预(灌装机停电,HVAC正常运行),如果是一个可预见的发生概率较高的情况,我认为还是作为一个纠正性干预,可能是个合理的行为。当然你不做,也最好有一些justification说明和评估。


